Pharmaceutical Intermediates Synthesis Expert
Focus on R&D and Production of THIAMINE HYDROCHLORIDE and related derivatives

Advantageous Products List
-
THIAMINE HYDROCHLORIDE
 CAS:70-16-6 Package:KG
CAS:70-16-6 Package:KG -
ThiaMine IMpurity C
CAS:7275-24-3 Pakage:KG
-
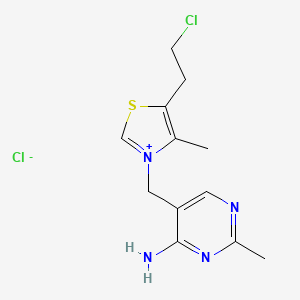
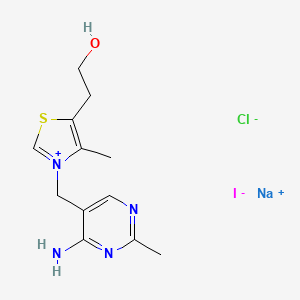
5-[[5-(2-chloroethyl)-4-methyl-1,3-thiazol-3-ium-3-yl]methyl]-2-methylpyrimidin-4-amine;chloride
CAS:13471-78-8 Pakage:KG
-
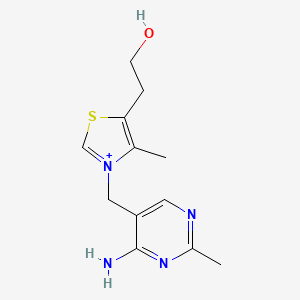
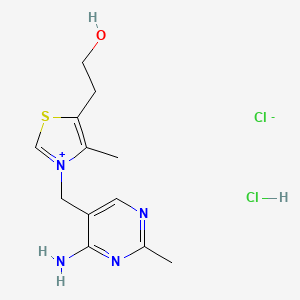
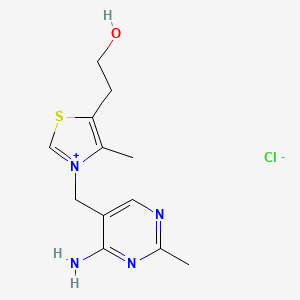
3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-5-(2-hydroxyethyl)-4-methyl-1,3-thiazol-3-ium chloride hydrochloride
CAS:67-03-8 Pakage:KG
-
Vitamin b1
CAS:59-43-8 Pakage:KG
-
Thiamine nitrate
CAS:532-43-4 Pakage:KG
-
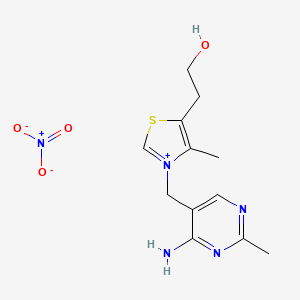
sodium;2-[3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-4-methyl-1,3-thiazol-3-ium-5-yl]ethanol;chloride;iodide
CAS:53571-49-6 Pakage:KG
-
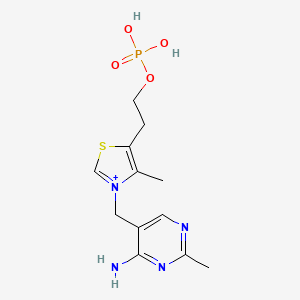
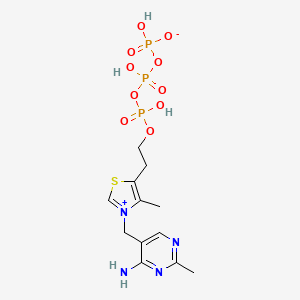
3-[(4-Amino-2-methyl-5-pyrimidinyl)methyl]-4-methyl-5-[2-(phosphonooxy)ethyl]thiazolium
CAS:10023-48-0 Pakage:KG
-
THIAMINE MONOPHOSPHATE CHLORIDE
CAS:532-40-1 Pakage:KG
-
THIAMINE MONOPHOSPHATE CHLORIDE*DIHYDRAT E
CAS:273724-21-3 Pakage:KG
-
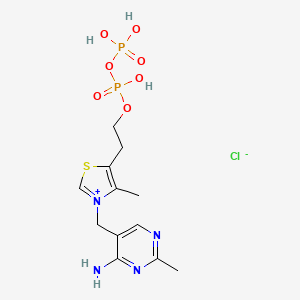
3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-5-(2-{[hydroxy(phosphonooxy)phosphoryl]oxy}ethyl)-4-methyl-1,3-thiazol-3-ium chloride
CAS:154-87-0 Pakage:KG
-
Benzyl 3-chloro-4-methylbenzoate
CAS:2056110-37-1 Pakage:KG
For more products? Try the search!

10+ years accumulated experiences
in synthesis from Impurities to Intermediates
40 + years of experience in complex chemical synthesis with professional technology originated from USA.
Focusing chemicals with high complexity.
The unique RMP accredditted by both ISO 17034 and CNAS-CLO4 in the world.
National high-tech enterprise
5000+m² R&D center
70+ experts with Dr. Degree
150 R&D instruments
18 patents

To provide customer with high quality services under an intact quality management policy.
Cato has established a complete quality management system and successively obtained ISO9001, ISO17034, CNAS and other international certifications base on 10+ years of continuous technological innovation and experience accumulation.

Introduced 150+ advanced equipment
for better quality assurance
The emphasis on quality is the foundation for our survival and development,
and the most basic requirement for the products and services we provide.
CATO has established a scientific, strict and comprehensive quality prevention
and assurance system in accordance with international quality standards.
Through advanced testing equipment such as GC-MS, chromatograph,
nuclear magnetic resonance, scanning electron microscopy,
ultraviolet rays, etc.,
quality management is implemented to every procedures of production and
operation activities such as raw materials, inventory, production, testing,
sales, verification, and training.
"All behaviors are controlled and traceable" is our criterion of works in CATO.
R&D team of 70+ PhD experts
-

Synthesis and separation Lab
Engaged in the R&D of chiral chromatographic synthesis and separation technology, and applied to separation and purification of compounds. Meantime, taking organic synthesis methodology as the innovation driving force, established library of chiral compounds with diverse structures.LEARN MORE
-

Synthetic routes designing Lab
The synthesis process strictly developed and optimized from laboratory to small-scale trial production and pilot production controls the micro impurity content in the mass production of intermediates, improve the yield rate, and find the best reaction conditions and treatment methods suitable for the production of industrial-grade raw materials.LEARN MORE
-

KG lever lab
A Multi-purpose, small-scale pharmaceutical preparation platform, to verify and produce non-sterile intermediates which is used for product verification, pilot scale-up, which can meet the requirements of particle size, crystal form, residue and solubility of specific projects.LEARN MORE
-

Synthesis Lab of intermediates
The lab can independently design the route of intermediate synthesis and process amplification, realize various complex conditional reactions and obtain the accurate structural confirmation in the reaction process, also the lab is capable for the H-NMR\C-NMR\F-NMR and various two-dimensional spectrum detection to ensure the accuracy of structural confirmation of multi chiral central compounds.LEARN MORE

1000+ successful projects
With capabilities to synthesize complex chemicals
So far, Cato has served more than 500 companies around the world mainly in the fields of pharmaceutical and biotechnology. The types of projects cover the whole cycle from preclinical to commercialization. By 2020, Cato has perfectly completed more than 1000 projects. CATO masters core technologies in critical technological areas such as high-end fluorine chemistry, asymmetric chiral synthesis, glycoside and biological enzyme technology, and continuous micro-reactors.

Cato is committed to building a high-level one-stop biomedical service platform to provide innovative process research, development and production services of intermediates, APIs and preparations for new drug R & D enterprises. With stable raw material supply management, Cato can continuously supply intermediates from gram level, kilogram level to ton level, and support new drug research, development till the commercialization stage.
Q&A
-
Support audit service? Yes, please make an appointment in advance, our address: No.179 BASIGO Park,Guangpu Rd East,Huangpu Dist,Guangzhou
-
Lead time: Normally the products are available in stock and can be shipped before 4:00 on the same day once the order is placed. Some products are made according to orders, and the delivery time will be depending on the production schedule of the workshop
-
Provide more spectrums? yes, we can carry out extra tests like water content, UV,IR,HNMR,CNMR, Nuclear magnetic two-dimensional spectroscopy, optical rotation detection, element analysis, TGA analysis and residue analysis and other related tests to support individual needs

-
Provide samples? Yes, samples can be provided free of charge for low value products, and small packaging specifications can be purchased for high value products
-
Production Capacity: at present, the production equipment is mainly performed by the laboratory and pilot line, with a maximum daily output of 1.5 tons.
-
Technical direction:Currently CATO mainly engages in the synthesis of pharmaceutical impurities and other reference standard products with core technologies for chirality, fluorine chemistry, and heterocycles.
-
Support audit service? Yes, please make an appointment in advance, our address: No.179 BASIGO Park,Guangpu Rd East,Huangpu Dist,Guangzhou
-
Lead time: Normally the products are available in stock and can be shipped before 4:00 on the same day once the order is placed. Some products are made according to orders, and the delivery time will be depending on the production schedule of the workshop
-
Provide more spectrums? yes, we can carry out extra tests like water content, UV,IR,HNMR,CNMR, Nuclear magnetic two-dimensional spectroscopy, optical rotation detection, element analysis, TGA analysis and residue analysis and other related tests to support individual needs
-
Provide samples? Yes, samples can be provided free of charge for low value products, and small packaging specifications can be purchased for high value products.
-
Production Capacity: at present, the production equipment is mainly performed by the laboratory and pilot line, with a maximum daily output of 1.5 tons.
-
Technical direction:Currently CATO mainly engages in the synthesis of pharmaceutical impurities and other reference standard products with core technologies for chirality, fluorine chemistry, and heterocycles.


-
Support audit service? Yes, please make an appointment in advance, our address: No.179 BASIGO Park,Guangpu Rd East,Huangpu Dist,Guangzhou
-
Lead time: Normally the products are available in stock and can be shipped before 4:00 on the same day once the order is placed. Some products are made according to orders, and the delivery time will be depending on the production schedule of the workshop
-
Provide more spectrums? yes, we can carry out extra tests like water content, UV,IR,HNMR,CNMR, Nuclear magnetic two-dimensional spectroscopy, optical rotation detection, element analysis, TGA analysis and residue analysis and other related tests to support individual needs
-
Provide samples? Yes, samples can be provided free of charge for low value products, and small packaging specifications can be purchased for high value products.
-
Production Capacity: at present, the production equipment is mainly performed by the laboratory and pilot line, with a maximum daily output of 1.5 tons.
-
Technical direction:Currently CATO mainly engages in the synthesis of pharmaceutical impurities and other reference standard products with core technologies for chirality, fluorine chemistry, and heterocycles.
Why CATO can achieve 80% market share in China?

5000+
m² R&D center

150+
R&D instruments

120+
Fume hoods

70+
experts with Dr. Degree

5000+
m² R&D center

150+
R&D instruments

120+
Fume hoods

70+
experts with Dr. Degree
Technical
Cooperation
In addition to our own independent R&D, CATO also closely cooperated with well-known institutions giving full play to the advantages of production, education, and research interaction to improve research and development efficiency. At the same time, it also greatly improved the speed and intensity of the company's technological innovation, and provide strong technical support.
Ready to discuss more about your demands?
Contact US